Atmospheric pressure varies so much, you would have to have weather station one yard apart from each other to get a truly accurate analysis. Changes in pressure result from a change in the quantity of mass over a given point. This may be the result of the actual removal/addition of mass or a change in the density of the atmosphere by differential heating/cooling. The scientific formula for pressure is P=F/A.
Pressure is the driving force of the weather phenomena. Pressure gradient force causes the wind to blow. Pressure has an effect on temperature. Below is a detailed description of measurements of atmospheric pressure to be understood from a meteorological standpoint.
Pressure is inversely proportional to area, when force is assumed constant.

Measuring Atmospheric Pressure
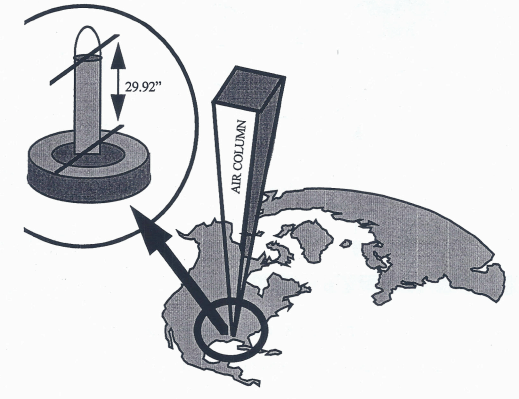
Mercurial barometer. Pressure is measured as the height of a column of mercury in an evacuated tube. An increase in pressure causes the height of the mercury to increase. Pressure is measured in millimeters or inches of mercury, which can be converted to pressure units. 29.92 in of mercury is equivalent to 1013.2 mb (1 mb = 0.03 in).

The scientific unit of pressure is the Pascal (Pa) named after Blaise Pascal (1623-1662). One pascal equals 0.01 millibar or 0.00001 bar. Meteorology has used the millibar for air pressure since 1929.
When the change to scientific unit occurred in the 1960’s many meteorologists preferred to keep using the magnitude they are used to and use a prefix “hecto” (h), meaning 100.
Therefore, 1 hectopascal (hPa) equals 100 Pa which equals 1 millibar. 100,000 Pa equals 1000 hPa which equals 1000 millibars.
The end result is although the units we refer to in meteorology may be different, their numerical value remains the same. For example the standard pressure at sea-level is 1013.25 millibars and 1013.25 hPa.
The atoms and molecules that make up the various layers in the atmosphere are constantly moving in random directions. Despite their tiny size, when they strike a surface they exert a force on that surface in what we observe as pressure.
Each molecule is too small to feel and only exerts a tiny bit of force. However, when we sum the total forces from the large number of molecules that strike a surface each moment, then the total observed
pressure can be considerable.
Air pressure can be increased (or decreased) one of two ways. First, simply adding molecules to any particular container will increase the pressure. A larger number of molecules in any particular container will increase the number of collisions with the container’s boundary which is observed as an increase in pressure.
A good example of this is adding (or subtracting) air in an automobile tire. By adding air, the number of molecules increase as well the total number of the collisions with the tire’s inner boundary. The increased number of collisions forces the tire’s pressure increase to expand in size.
The second way of increasing (or decreasing) is by the addition (or subtraction) of heat. Adding heat to any particular container can transfer energy to air molecules. The molecules therefore move with increased velocity striking the container’s boundary with greater force and is observed as an increase in pressure.

Since molecules move in all directions, they can even exert air pressure upwards as they smash into object from underneath. In the atmosphere, air pressure can be exerted in all directions.
In the International Space Station, the density of the air is maintained so that it is similar to the density at the earth’s surface. Therefore, the air pressure is the same in the space station as the earth’s surface (14.7 pounds per square inch).
Back on Earth, as elevation increases, the number of molecules decreases and the density of air therefore is less, meaning a decrease in air pressure. In fact, while the atmosphere extends more than 15 miles (24 km) up, one half of the air molecules in the atmosphere are contained within the first 18,000 feet (5.6 km).
Because of this decrease in pressure with height, it makes it very hard to compare the air pressure at ground level from one location to another, especially when the elevations of each site differ. Therefore, to give meaning to the pressure values observed at each station, we convert the station air pressures reading to a value with a common denominator.
The common denominator we use is the sea-level elevation. At observation stations around the world the air pressure reading, regardless of the observation station elevation, is converted to a value that would be observed if that instrument were located at sea level.
The two most common units in the United States to measure the pressure are “Inches of Mercury” and “Millibars”. Inches of mercury refers to the height of a column of mercury measured in hundredths of inches. This is what you will usually hear from the NOAA Weather Radio or from your favorite weather or news source. At sea level, standard air pressure is 29.92 inches of mercury.
Millibars comes from the original term for pressure “bar”. Bar is from the Greek “báros” meaning weight. A millibar is 1/1000th of a bar and is approximately equal to 1000 dynes (one dyne is the amount of force it takes to accelerate an object with a mass of one gram at the rate of one centimeter per second squared). Millibar values used in meteorology range from about 100 to 1050. At sea level, standard air pressure in millibars is 1013.2. Weather maps showing the pressure at the surface are drawn using millibars.

Although the changes are usually too slow to observe directly, air pressure is almost always changing. This change in pressure is caused by changes in air density, and air density is related to temperature.
Warm air is less dense than cooler air because the gas molecules in warm air have a greater velocity and are farther apart than in cooler air. So, while the average altitude of the 500 millibar level is around 18,000 feet (5,600 meters) the actual elevation will be higher in warm air than in cold air.


The most basic change in pressure is the twice daily rise and fall in due to the heating from the sun. Each day, around 4 a.m./p.m. the pressure is at its lowest and near its peak around 10 a.m./p.m. The magnitude of the daily cycle is greatest near the equator decreasing toward the poles.
On top of the daily fluctuations are the larger pressure changes as a result of the migrating weather systems. These weather systems are identified by the blue H’s and red L’s seen on weather maps.

How are changes in weather related to changes in pressure?
From his vantage point in England in 1848, Rev. Dr. Brewer wrote in his A Guide to the Scientific Knowledge of Things Familiar the following about the relation of pressure to weather:
The FALL of the barometer (decreasing pressure)
- In very hot weather, the fall of the barometer denotes thunder. Otherwise, the sudden falling of the barometer denotes high wind.
- In frosty weather, the fall of the barometer denotes thaw.
- If wet weather happens soon after the fall of the barometer, expect but little of it.
- In wet weather if the barometer falls expect much wet.
- In fair weather, if the barometer falls much and remains low, expect much wet in a few days, and probably wind.
- The barometer sinks lowest of all for wind and rain together; next to that wind, (except it be an east or north-east wind).
The RISE of the barometer (increasing pressure)
- In winter, the rise of the barometer presages frost.
- In frosty weather, the rise of the barometer presages snow.
- If fair weather happens soon after the rise of the barometer, expect but little of it.
- In wet weather, if the mercury rises high and remains so, expect continued fine weather in a day or two.
- In wet weather, if the mercury rises suddenly very high, fine weather will not last long.
- The barometer rises highest of all for north and east winds; for all other winds it sinks.
The barometer UNSETTLED (unsteady pressure)
- If the motion of the mercury be unsettled, expect unsettled weather.
- If it stands at “MUCH RAIN” and rises to “CHANGEABLE” expect fair weather of short continuance.
- If it stands at “FAIR” and falls to “CHANGEABLE”, expect foul weather.
- Its motion upwards, indicates the approach of fine weather; its motion downwards, indicates the approach of foul weather.
These pressure observations hold true for many other locations as well but not all of them. Storms that occur in England, located near the end of the Gulf Stream, bring large pressure changes. In the United States, the largest pressure changes associated with storms will generally occur in Alaska and northern half of the continental U.S. In the tropics, except for tropical cyclones, there is very little day-to-day pressure change and none of the rules apply.
Recent Posts
Determining Severe Weather Based On Stability Indexes and Upper-Level Winds
There are several weather products used to determine the possibility of severe weather for an area. The most common and misunderstood by many weather enthusiasts is the Skew-T chart and the upper-air...
Tornado Basics, Severe Weather Preparation, & The Enhanced Fujita scale
Earth's weather can produce various kinds of windstorms which include waterspouts, dust devils and tornadoes. Although they have the common features of a column of rotating air, they are actually...